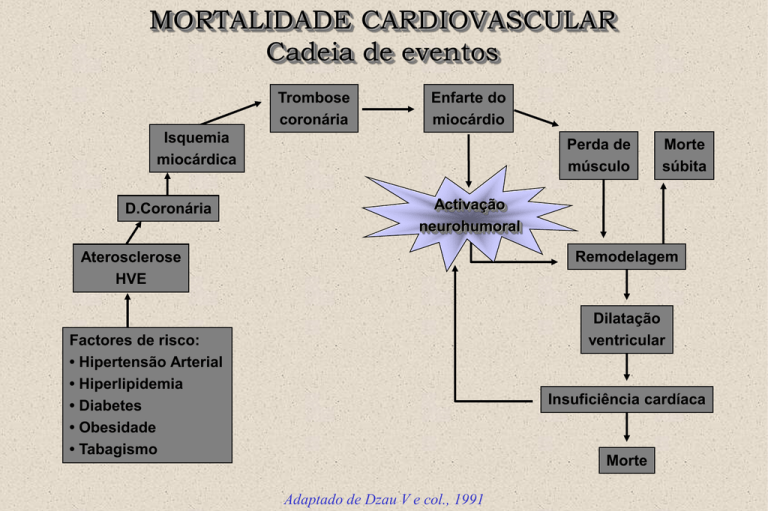
MORTALIDADE CARDIOVASCULAR
Cadeia de eventos
Trombose
coronária
Enfarte do
miocárdio
Isquemia
miocárdica
D.Coronária
Perda de
músculo
Morte
súbita
Activação
neurohumoral
Remodelagem
Aterosclerose
HVE
Dilatação
ventricular
Factores de risco:
• Hipertensão Arterial
• Hiperlipidemia
• Diabetes
• Obesidade
• Tabagismo
Insuficiência cardíaca
Morte
Adaptado de Dzau V e col., 1991
INSUFICIÊNCIA CARDÍACA
INSUFICIÊNCIA CARDÍACA
• SINDROME COMPLEXO, COM ETIOLOGIAS
VÁRIAS
• ATINGE CERCA DE 20 MILHÕES DE
PESSOAS EM TODO O MUNDO
• NÚMERO DE CASOS SOBE EXPONENCIALMENTE SOBRETUDO NO IDOSO
10% DOS IDOSOS COM IDADE > 75 ANOS
TÊM INSUFICIÊNCIA CARDÍACA
INSUFICIÊNCIA CARDÍACA
O PROGNÓSTICO É MAU
• A MORBILIDADE É MUITO SIGNIFICATIVA
• AS ADMISSÕES HOSPITALARES SÃO
MUITO FREQUENTES ( CAUSA MAIS
HABITUAL DE INTERNAMENTO EM IDADE
> 65 ANOS)
INSUFICIÊNCIA CARDÍACA
PORQUE É QUE A INS. CARDÍACA AUMENTA?
• POPULAÇÃO MAIS ENVELHECIDA
• SUCESSO DA TERAPÊUTICA NA MELHORIA
DA SOBREVIVÊNCIA PÓS-EAM (trombólise ou
outras medidas)
• AUMENTO NA DURAÇÃO DA SOBREVIDA
DOS DOENTES COM INSUFICIÊNCIA
CARDÍACA
DEFINITION
“The situation when the heart is
incapable of maintaining a cardiac
output adequate to accommodate
metabolic requirements and the
venous return."
E. Braunwald
New York Heart Association
Functional Classification
Class I:
No symptoms with ordinary activity
Class II:
Slight limitation of physical activity. Comfortable at rest,
but ordinary physical activity results in fatigue,
palpitation, dyspnea, or angina
Class III:
Marked limitation of physical activity. Comfortable at
rest, but less than ordinary physical activity results in
fatigue, palpitation, dyspnea, or anginal pain
Class IV:
Unable to carry out any physical activity without
discomfort. Symptoms of cardiac insufficiency may be
present even at rest
Severity of Heart Failure
Modes of Death
NYHA II
NYHA III
CHF
CHF
12%
Other
26%
59%
Sudden
Death
24%
64%
Other
15%
n = 103
Sudden
Death
n = 103
NYHA IV
CHF
Other
33%
56%
11%
Sudden
Death
n = 27
MERIT-HF Study Group. Effect of Metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomized intervention trial in
congestive heart failure (MERIT-HF). LANCET. 1999;353:2001-07.
Etiology of Heart Failure
What causes heart failure?
• The loss of a critical quantity of functioning myocardial
cells after injury to the heart due to:
–
–
–
–
–
–
–
Ischemic Heart Disease
Hypertension
Idiopathic Cardiomyopathy
Infections (e.g., viral myocarditis, Chagas’ disease)
Toxins (e.g., alcohol or cytotoxic drugs)
Valvular Disease
Prolonged Arrhythmias
Left Ventricular Dysfunction
• Systolic: Impaired contractility/ejection
– Approximately two-thirds of heart failure patients have systolic
dysfunction1
• Diastolic: Impaired filling/relaxation
30%
(EF > 40 %)
(EF < 40%)
70%
Diastolic Dysfunction
Systolic Dysfunction
1 Lilly, L. Pathophysiology of Heart Disease. Second Edition p 200
DETERMINANTS OF
VENTRICULAR FUNCTION
CONTRACTILITY
PRELOAD
AFTERLOAD
STROKE
VOLUME
- Synergistic LV contraction
- LV wall integrity
- Valvular competence
HEART
RATE
CARDIAC OUTPUT
Hemodynamic Basis for
Heart Failure Symptoms
Hemodynamic Basis for
Heart Failure Symptoms
LVEDP
Left Atrial Pressure
Pulmonary Capillary Pressure
Pulmonary Congestion
Left Ventricular Dysfunction
Systolic and Diastolic
• Symptoms
• Physical Signs
– Dyspnea on Exertion
– Basilar Rales
– Paroxysmal Nocturnal
Dyspnea
– Pulmonary Edema
– Tachycardia
– S3 Gallop
– Cough
– Pleural Effusion
– Hemoptysis
– Cheyne-Stokes Respiration
ICC - Fase de respostas compensatórias
Melhoria transitória
Activação
neurohumoral
Retenção H2O e sal
Vasoconstrição
Redistribuição fluxo
Inotropia +
Taquicardia
Disfunção
miocárdica
Melhoria transitória
Dilatação e
hipertrofia
Frank-Starling
< stress da parede
Compensatory Mechanisms
• Frank-Starling Mechanism
• Neurohormonal Activation
• Ventricular Remodeling
Compensatory Mechanisms
Frank-Starling Mechanism
a. At rest, no HF
b. HF due to LV systolic
dysfunction
c. Advanced HF
Compensatory Mechanisms
Neurohormonal Activation
Many different hormone systems are involved in
maintaining normal cardiovascular homeostasis,
including:
• Sympathetic nervous system (SNS)
• Renin-angiotensin-aldosterone system (RAAS)
• Vasopressin (a.k.a. antidiuretic hormone, ADH)
Equilíbrio sistemas neuro-humorais
reguladores perfusão
Vasodilatores
Natriuréticos
Anti-proliferativos
Anti-inflamatórios
Antitrombogénicos
Dopamina
ANF, BNF, CNF
Adrenomedulina
Prostaciclina
Bradicinina
NO
Vasoconstritores
Anti-natriuréticos
Pró-proliferativos
Pró-inflamatórios
Trombogénicos
Angiotensina 2
Aldosterona
Adrenalina
Noradrenalina
Endotelina
VSP
TBX A2
Ubaína
Neurohormonal Responses to Impaired
Cardiac Performance
Initially Adaptive
Response
Short-Term
Effects
Salt and Water Retention
Augments Preload
Vasoconstriction
Maintains BP for perfusion of
vital organs
Sympathetic Stimulation
Increases HR and ejection
Jaski, B, MD: Basics of Heart Failure: A Problem Solving Approach
Sympathetic Activation in Heart Failure
CNS sympathetic outflow
Cardiac sympathetic
activity
1receptors
2receptors
Sympathetic
activity to kidneys
+ peripheral vasculature
1receptors
Myocardial toxicity
Increased arrhythmias
1-
Activation
of RAS
Vasoconstriction
Sodium retention
Disease progression
Packer. Progr Cardiovasc Dis. 1998;39(suppl I):39-52.
1-
1 and 2 receptor densities in the
failing and non-failing heart
Receptor density (ƒ mol/mg)
80
Non-failing
Failing
60
*p<0.05
**p=NS
40
*
**
20
0
1
2
Compensatory Mechanisms:
Renin-Angiotensin-Aldosterone (RAAS)
Angiotensinogen
Renin
Angiotensin I
Angiotensin
Converting
Enzyme
Angiotensin II
Na+ retention
AT I receptor
Vasoconstriction
Oxidative Stress
Cell Growth
Vascular remodeling
LV remodeling
Proteinuria
Other Neurohormones
• Natriuretic Peptides: Three known types
– Atrial Natriuretic Peptide (ANP)
• Predominantly found in the atria
• Diuretic and vasodilatory properties
– Brain Natriuretic Peptide (hBNP)
• Predominantly found in the cardiac ventricles
• Diuretic and vasodilatory properties
– C-type Natriuretic Peptide (CNP)
• Predominantly found in the central nervous system
• Limited natriuretic and vasodilatory properties
Pharmacological Actions of hBNP
Hemodynamic
(balanced vasodilation)
R I S S
D
S
M
S
K
G
R
L
G
H
G
F
R
C
R
C S S
K V L
G
K
P
M V
S
Q G S
• veins
• arteries
• coronary arteries
Neurohormonal
aldosterone
norepinephrine
Renal
diuresis & natriuresis
Abraham WT and Schrier RW, 1994
Endothelium-Derived Vasoactive
Substances
Produced by a thin lining of cells within the arteries and veins
called the endothelium
Endothelium-derived relaxing factors (EDRF) – Vasodilators:
• Nitric Oxide (NO)
• Bradykinin
• Prostacyclin
Endothelium-derived constricting factors (EDCF) –
Vasoconstrictors:
• Endothelin I
Mediators of Heart Failure
Cytokines
• Small protein molecules produced by a variety of tissues
and cells
• Negative inotropes
• Elevated levels associated with worse clinical outcomes
• Examples:
– Tumor necrosis factor (TNF)-alpha
– Interleukin 1-alpha
– Interleukin-2
– Interleukin-6
– Interferon-alpha
Neurohormonal Responses to Impaired
Cardiac Performance
Initially Adaptive, Deleterious if Sustained
Long-Term Effects
Response
Short-Term
Effects
Salt and Water Retention
Augments Preload
Pulmonary Congestion,
Anasarca
Vasoconstriction
Maintains BP for perfusion of
vital organs
Exacerbates pump dysfunction
(excessive afterload), increases
cardiac energy expenditure
Sympathetic Stimulation
Increases HR and ejection
Increases energy expenditure
Jaski, B, MD: Basics of Heart Failure: A Problem Solving Approach
ICC - Fase de descompensação
agravamento
Disfunção
miocárdica
Dilatação ventricular
Remodelagem
Activação
neurohumoral
Stress oxidativo
Citocinas
Apoptose
Mitogénese
Proliferação celular
Alterações estruturais, miocárdio, tecido
conjuntivo, vasos
Perda de miócitos, necrose e
fibrose
Dilatação e
hipertrofia
General Measures
Lifestyle Modifications:
Medical Considerations:
• Weight reduction
• Treat HTN, hyperlipidemia, diabetes,
arrhythmias
• Discontinue smoking
• Coronary revascularization
• Avoid alcohol and other
cardiotoxic substances
• Anticoagulation
• Exercise
• Immunization
• Sodium restriction
• Daily weights
• Close outpatient monitoring
Objectivos terapêuticos
sobrevida
Morbilidade
Capacidade de Exercicio
Qualidade de vida
Alterações Neurohormonais
Progressão da CHF
Sintomas
Tratamento da Ins. cardiaca
Diureticos e digitalicos
Vasodilatadores
Directos e nitratos
Inibidores da ECA
Antagonistas dos receptores AT1
Bloqueadores beta
Antiarrítmicos, anticoagulantes
Inibidores das fosfodiesterases redutores da
produçãode FNT e outras citocinas)
Ressincronização cardíaca
DRUGS
HEMODYNAMIC EFFECTS
Normal
I
Stroke
Volume
A
A+V
V
D
CHF
Ventricular Filling Pressure
Pharmacologic Management
Digoxin
• Enhances inotropy of cardiac muscle
• Reduces activation of SNS and RAAS
• Controlled trials have shown long-term digoxin therapy:
– Reduces symptoms
– Increases exercise tolerance
– Improves hemodynamics
– Decreases risk of HF progression
– Reduces hospitalization rates for decompensated HF
– Does not improve survival
Genina
OH
CH3
Tri-digitoxose (açucares)
o
CH3
CH 3
CH 3
o
HO
-
o
- o-
OH
CH 3
o
- oEsteroide
Figura 1. Estrutura da digoxina, protótipo dos digitálicos
Lactona
DIGOXIN
Na-K ATPase
Na+
K+
K+ Na+
Na-Ca Exchange
Na+
Myofilaments
Ca++
Ca++
CONTRACTILITY
Figura 2. Efeitos inotrópicos e neurais dos digitálicos
Efeito simpático-inibidor
aferências
doses
terapêuticas
Digitálicos
Estimulação vagal
2K +
3Na +
> saída
de sódio
Trocador
Na+/Ca2+
Estimulação simpática
Doses
tóxicas
Taquiarritmias
> sódio
intracelular
> Ca 2+
intracelular
EFEITO INOTRÓPICO POSITIVO
Aumento Ca2+
intracelular
Adaptado de Opie, 1990
Normal Conduction Pathway in the
Heart and the ECG
Sinoatrial (SA) Node
Atrioventricular (AV) Node
Left Bundle Branches
Right Bundle Branch
Purkinje Fibers
P
T
QRS
P=
Atrial Depolarization
QRS = Ventricular Depolarization
T=
Ventricular Repolarization
DIGOXIN
PHARMACOKINETIC PROPERTIES
Oral absorption (%)
Protein binding (%)
Volume of distribution (l/Kg)
Half life
Elimination
Onset (min)
i.v.
oral
Maximal effect (h)
i.v.
oral
Duration
Therapeutic level (ng/ml)
60 - 75
25
6 (3-9)
36 (26-46) h
Renal
5 - 30
30 - 90
2-4
3-6
2 - 6 days
0.5 - 2
DIGOXIN
HEMODYNAMIC EFFECTS
Cardiac output
LV ejection fraction
LVEDP
Exercise tolerance
Natriuresis
Neurohormonal activation
DIGOXIN
NEUROHORMONAL EFFECTS
Plasma Noradrenaline
Peripheral nervous system activity
RAAS activity
Vagal tone
Normalizes arterial baroreceptors
DIGOXIN
LONG TERM EFFECTS
Survival similar to placebo
Fewer hospital admissions
More serious arrhythmias
More myocardial infarctions
DIGOXIN
CLINICAL USES
AF with rapid ventricular response
CHF refractory to other drugs
Other indications?
Can be combined with other drugs
DIGOXIN
CONTRAINDICATIONS
ABSOLUTE:
- Digoxin toxicity
RELATIVE
- Advanced A-V block without pacemaker
- Bradycardia or sick sinus without PM
- PVC’s and TV
- Marked hypokalemia
- W-P-W with atrial fibrillation
DIGOXIN TOXICITY
CARDIAC MANIFESTATIONS
ARRHYTHMIAS :
- Ventricular (PVCs, TV, VF)
- Supraventricular (PACs, SVT)
BLOCKS:
- S-A and A-V blocks
CHF EXACERBATION
DIGOXIN TOXICITY
EXTRACARDIAC MANIFESTATIONS
GASTROINTESTINAL:
- Nausea, vomiting, diarrhea
NERVOUS:
- Depression, disorientation, paresthesias
VISUAL:
- Blurred vision, scotomas and yellow-green
vision
HYPERESTROGENISM:
- Gynecomastia, galactorrhea
POSITIVE INOTROPES
CARDIAC GLYCOSIDES
SYMPATHOMIMETICS
Catecholamines
ß-adrenergic agonists
PHOSPHODIESTERASE INHIBITORS
Amrinone
Enoximone
Others
Milrinone
Piroximone
ß-ADRENERGIC STIMULANTS
CLASSIFICATION
B1 Stimulants
Increase contractility
Dobutamine Doxaminol Xamoterol
Butopamine Prenalterol Tazolol
B2 Stimulants
Produce arterial vasodilatation and reduce SVR
Pirbuterol Rimiterol Tretoquinol Terbutaline Soterenol
Carbuterol Fenoterol Salbutamol Salmefamol Quinterenol
Mixed
Dopamine
DOPAMINE AND DOBUTAMINE
EFFECTS
DA (µg / Kg / min)
Dobutamine
<2
DA1 / DA2
2-5
ß1
>5
ß1 +
ß1
Contractility
±
++
++
++
Heart Rate
±
+
++
±
Arterial Press.
±
+
++
++
++
+
±
+
-
±
++
±
Receptors
Renal perfusion
Arrhythmia
POSITIVE INOTROPES
CONCLUSIONS
May increase mortality
Safer in lower doses
Use only in refractory CHF
NOT for use as chronic therapy
DIURETICS
Thiazides
Inhibit active exchange of Cl-Na
in the cortical diluting segment of the
ascending loop of Henle
Cortex
K-sparing
Inhibit reabsorption of Na in the
distal convoluted and collecting tubule
Loop diuretics
Medulla
Inhibit exchange of Cl-Na-K in
the thick segment of the ascending
loop of Henle
Loop of Henle
Collecting tubule
THIAZIDES
MECHANISM OF ACTION
Excrete 5 - 10% of filtered Na+
Elimination of K
Inhibit carbonic anhydrase:
increase elimination of HCO3
No dose - effect relationship
LOOP DIURETICS
MECHANISM OF ACTION
Excrete 15 - 20% of filtered Na+
Elimination of K+, Ca+ and Mg++
Resistance of afferent arterioles
-
Cortical flow and GFR
-
Release renal PGs
-
NSAIDs may antagonize diuresis
K-SPARING DIURETICS
MECHANISM OF ACTION
Eliminate < 5% of filtered Na+
Inhibit exchange of Na+ for K+ or H+
Spironolactone = competitive
antagonist for the aldosterone receptor
Amiloride and triamterene block
Na+ channels controlled by aldosterone
DIURETIC EFFECTS
Volume and preload
Improve symptoms of congestion
No direct effect on CO, but
excessive preload reduction may
Improves arterial distensibility
Neurohormonal activation
Levels of NA, Ang II and ARP
Exception: with spironolactone
DIURETICS
ADVERSE REACTIONS
Thiazide and Loop Diuretics
Changes in electrolytes:
Volume
Na+, K+, Ca++, Mg++
metabolic alkalosis
Metabolic changes:
glycemia, uremia, gout
LDL-C and TG
Cutaneous allergic reactions
DIURETICS
ADVERSE REACTIONS
Thiazide and Loop Diuretics
Idiosyncratic effects:
Blood dyscrasia, cholestatic jaundice and
acute pancreatitis
Gastrointestinal effects
Genitourinary effects:
Impotence and menstrual cramps
Deafness, nephrotoxicity
(Loop diuretics)
Pharmacologic Management
Diuretics
• Used to relieve fluid retention
• Improve exercise tolerance
• Facilitate the use of other drugs indicated for heart failure
• Patients can be taught to adjust their diuretic dose based on
changes in body weight
• Electrolyte depletion a frequent complication
• Should never be used alone to treat heart failure
• Higher doses of diuretics are associated with increased
mortality
DIURETICS
ADVERSE REACTIONS
K-SPARING DIURETICS
Changes in electrolytes:
Na+,
K+, acidosis
Musculoskeletal:
Cramps, weakness
Cutaneous allergic reactions :
Rash, pruritis
VASODILATOR DRUGS
PRINCIPLES
Normal Contractility
Normal Contractility
CO
VV
Diminished
Contractility
PRELOAD
AV
Diminished
Contractility
AFTERLOAD
VASODILATORS
CLASSIFICATION
VENOUS
Nitrates
Molsidomine
MIXED
Calcium antagonists
-adrenergic Blockers
ACEI
Angiotensin II inhibitors
K+ channel activators
Nitroprusside
Arterial
Vasodilatation
ARTERIAL
Minoxidil
Hydralazine
Venous
Vasodilatation
NITRATES
HEMODYNAMIC EFFECTS
1- VENOUS VASODILATATION
Preload
Pulmonary congestion
Ventricular size
Vent. Wall stress
MVO2
2- Coronary vasodilatation
Myocardial perfusion
3- Arterial vasodilatation
Afterload
4- Others
• Cardiac output
• Blood pressure
NITRATES
SURVIVAL
0.7
Placebo (273)
Prazosin (183)
Hz + ISDN (186)
0.6
0.5
PROBABILITY
OF
DEATH
0.4
0.3
0.2
0.1
0
VHefT-1
N Engl J Med 1986;314:1547
0
6
12
18
24
MONTHS
30
36
42
NITRATES
TOLERANCE
" Decrease in the effect of a drug
when administered in a long-acting form"
Develops with all nitrates
Is dose-dependent
Disappears in 24 h. after stopping the drug
Tolerance can be avoided
- Using the least effective dose
- Creating discontinuous plasma levels
NITRATES
TOLERANCE
Can be avoided or minimized
- Intermittent administration
- Use the lowest possible dose
- Intersperse a nitrate-free interval
Allow peaks and valleys in plasma levels
- Vascular smooth muscle recovers its
nitrate sensitivity during the nadirs
- Patches: remove after 8-10 h
NITRATES
TOLERANCE
H
A
L
F
L
I
F
E
s.l. NTG
ISDN
I 5-MN
Percutaneous NTG
T
O
L
E
R
A
N
C
E
NITRATES
CONTRAINDICATIONS
Previous hypersensitivity
Hypotension ( < 80 mmHg)
AMI with low ventricular filling pressure
1st trimester of pregnancy
WITH CAUTION:
ž Constrictive pericarditis
ž Intracranial hypertension
ž Hypertrophic cardiomyopathy
NITRATES
CLINICAL USES
Pulmonary congestion
Orthopnea and paroxysmal nocturnal
dyspnea
CHF with myocardial ischemia
In acute CHF and pulmonary edema:
NTG s.l. or i.v.
Pharmacologic Management
ACE Inhibitors
• Blocks the conversion of angiotensin I to angiotensin II;
prevents functional deterioration
• Recommended for all heart failure patients
• Relieves symptoms and improves exercise tolerance
• Reduces risk of death and decreases disease progression
• Benefits may not be apparent for 1-2 months after initiation
ACEI
MECHANISM OF ACTION
VASOCONSTRICTION
ALDOSTERONE
VASOPRESSIN
SYMPATHETIC
VASODILATATION
PROSTAGLANDINS
Kininogen
tPA
Kallikrein
Angiotensinogen
RENIN
Angiotensin I
A.C.E.
ANGIOTENSIN II
Inhibitor
BRADYKININ
Kininase II
Inactive Fragments
Retenção de Na+
Libertação de
Aldosterona
e ET-1
Hipertrofia cardíaca
Fibrose intersticial
Pro-ateromatose
Efeitos centrais
Angiotensina II
Estimulação de
protooncogenes
Estimulação
simpática
Hipertrofia/Hiperplasia
Remodelagem vascular
Inflamação/procoagulabilidade
Stress oxidativo
ACEI
HEMODYNAMIC EFFECTS
Arteriovenous Vasodilatation
-
PAD, PCWP and LVEDP
SVR and BP
CO and exercise tolerance
No change in HR / contractility
MVO2
Renal, coronary and cerebral flow
Diuresis and natriuresis
ACEI
ADVANTAGES
Inhibit LV remodeling post-MI
Modify the progression of chronic CHF
Survival
Hospitalizations
- Improve the quality of life
In contrast to others vasodilators,
do not produce neurohormonal activation
or reflex tachycardia
Tolerance to its effects does not develop
ACEI SURVIVAL
0.8
0.7
Placebo
0.6
PROBABILITY
OF
DEATH
p< 0.001
0.5
0.4
p< 0.002
0.3
Enalapril
0.2
0.1
CONSENSUS
N Engl J Med 1987;316:1429
0
0
1
2
3
4
5
6
7
MONTHS
8
9
10
11
12
ACEI
INDICATIONS
Clinical cardiac insufficiency
- All patients
Asymptomatic ventricular
dysfunction
- LVEF < 35 %
ACEI
UNDESIRABLE EFFECTS
Inherent in their mechanism of action
- Hypotension
- Hyperkalemia
- Angioneurotic edema
- Dry cough
- Renal Insuff.
Due to their chemical structure
- Cutaneous eruptions
- Neutropenia,
thrombocytopenia
- Digestive upset
- Dysgeusia
- Proteinuria
ACEI
CONTRAINDICATIONS
Renal artery stenosis
Renal insufficiency
Hyperkalemia
Arterial hypotension
Intolerance (due to side effects)
IECAS
VANTAGENS
DESVANTAGENS
- reduzem mortalidade
- efeitos acessórios
e morbilidade
(tosse /angioedema)
- melhoram sintomas
- aumentam tolerância
ao esforço
- reduzem hospitalizações
- eficazes na I C assintomática
NEJM 1987: 316;1429
NEJM 1991: 325; 303
JAMA 1988:259; 539
NEJM 1991:325;293
ANN INTERN MED 1992:117 ; 234
Mortality trials with ACE inhibitors in heart
failure
Trial
Treatment
Treatments
n
Enalapril
253
Placebo
V-HeFT-II
2 years
Enalapril
(treatment)
41 months
Enalapril
Placebo
ISDN=isosorbide dinitrate; Hydral=hydralazine
26
0.002
44
804
ISDN/Hydral
SOLVD
p value
(%)
duration
CONSENSUS-1 6 months
Mortality
18
0.016
25
2569
35
40
0.004
ACE inhibitor trials in heart
failure following AMI
n
Mortality p value
(%)
Trial
Treatment Treatments
duration
AIRE
15 months
Ramipril
Placebo
2006
17
23
0.002
SAVE
42 months
Captopril
Placebo
2231
20
25
0.019
Trandolapril 1749
Placebo
35
42
0.001
TRACE
4 years
Estudos de mortalidade com In ECA na
insuficiência cardíaca e E. miocárdio
•
32 estudos randomizados em 7105 doentes
•
Redução significativa da mortalidade global
– odds ratio 0.77 (95% C.I. 0.67-0.88); p<0.001
•
Redução significativa da mortalidade + hospitalizações por insuficiência cardíaca de 25%
– odds ratio 0.65 (95% C.I. 0.57-0.74); p<0.001
•
Maiores benefícios em doentes com maior
deterioração da função cardíaca
SISTEMA RENINA - ANGIOTENSINA
Angiotensinogénio
Cathepsin G,
Calicreína,
Tonina,
Tripsina
Renina
Angiotensina I
Chymase
CAGE,
Calicreína, ...
Bradicinina
ECA
Peptídeos
inactivos
Angiotensina II
-
?
Antagonistas
(AT1)
Receptores BK 2
ALDO
Vasoconstrição
Antinatriurese
Proliferação celular
Inflamação, aterogenese
Hipercoagulação
Receptores AT1
Receptores AT2
Vasodilatação
Efeito antiproliferativo
antiaterogenico
?
Óxido nítrico
PG
ANGIOTENSIN II INHIBITORS
MECHANISM OF ACTION
RENIN
Angiotensin I
Angiotensinogen
ACE
Other paths
ANGIOTENSIN II
AT1
RECEPTOR
BLOCKERS
AT1
Vasoconstriction
RECEPTORS
Proliferative
Action
AT2
Vasodilatation
Antiproliferative
Action
Pharmacologic Management
Angiotensin Receptor Blockers (ARBs)
• Block AT1 receptors, which bind circulating angiotensin II
• Examples: irbesartan, valsartan, candesartan, losartan
• Should not be considered equivalent or superior to ACE
inhibitors
• In clinical practice, ARBs should be used to treat patients who
are ACE intolerant due to intractable cough or who develop
angioedema
Angiotensin II Receptors
AT1 receptor
AT2 receptor
• Vasoconstriction
• Vasodilation
• Growth Promotion
• Growth inhibition
• Anti-apoptotic
• Pro-apoptotic
• Pro-fibrotic
• ? Fibrosis
• Pro-thrombotic
• ? Thrombosis
• Pro-oxidant
• ? redox
AT1 RECEPTOR BLOCKERS
DRUGS
Losartan
Valsartan
Irbersartan
Candesartan
Competitive and selective
blocking of AT1 receptors
The ELITE-study
400
350
Losartan
Captopril
300
250
200
150
100
p = 0.075
p = 0.035
50
0
Number of
patients
Adverse
events
Death and
hospitalization
Death
Pitt et al. Lancet 1997: 349: 747-52
Losartan Heart Failure Survival Study
ELITE II
Study Design
60 years; NYHA II-IV; EF 40%
ACEI/AIIA naive or <7 days in 3 months prior to entry
Standard Rx (± Dig/Diuretics), -blocker stratification
Captopril
50 mg 3 times daily
(n=1574)
Event-driven
(Target 510 Deaths)
~2 years
Losartan
50 mg daily
(n=1578)
Primary Endpoint:
All-Cause Mortality
Secondary Endpoint: Sudden Cardiac Death and/or Resuscitated Arrest
Other Endpoints:
All-Cause Mortality/Hospitalizations
Safety and Tolerability
Losartan Heart Failure Survival Study – ELITE II
Mortality by Cause (Adjudicated)
% of Patients
15
Losartan (n=1578)
Captopril (n=1574)
10
5
0
Sudden
death
Heart
failure
MI
Stroke
Other
CV
Non-CV
INSUFICIÊNCIA CARDÍACA
VALOR DOS ANTAGONISTAS DA AII
• MELHORAM OS SINTOMAS, AUMENTAM A
QUALIDADE DE VIDA E AUMENTAM A
TOLERÂNCIA AO EXERCÍCIO
• REDUZEM A MORBILIDADE E O NÚMERO DE
HOSPITALIZAÇÕES.
• AUMENTAM A ADERÊNCIA AO TRATAMENTO (poucos efeitos secundários).
ÓPTIMA ALTERNATIVA AOS IECAS
CONCEITO DOS INIBIDORES DAS
VASOPEPTIDASES
- DUPLO BLOQUEIO DA NEP
e da ECA
Inibição das
vasopeptidases
NEP
ANP e peptideos
Análogos
Adrenomedulina
Vasodilatação
Excreção sódio
Efeitos antihipertróficos
ECA
BK
Angiotensina II
Vasoconstrição
Retenção sódio
Efeitos hipertróficos
Pressão arterial
Melhoria da performance cardíaca
Protecção dos orgãos-alvo
Omapatrilat: Survival Benefit
Cardiomyopathic hamsters
100
Survival (%)
80
60
146 d
221 d
290 d
40
20
Placebo
Omapatrilat
Captopril
0
40
Start treatment
80
120
160
200
240
280
Days of treatment
Trippodo et al. J Cardiovasc Pharmacol 1999;34:782
320
360
400
440
Pharmacologic Management
Aldosterone Antagonists
• Generally well-tolerated
• Shown to reduce heart failure-related morbidity and mortality
• Generally reserved for patients with NYHA Class III-IV HF
• Side effects include hyperkalemia and gynecomastia. Potassium and
creatinine levels should be closely monitored
• REDUÇÂO MORTALIDADE ASSOCIADOS AOS InECA
• (estudo RALES)
ALDOSTERONE INHIBITORS
Spironolactone
ALDOSTERONE
Competitive antagonist of the
aldosterone receptor
(myocardium, arterial walls, kidney)
Retention Na+
Retention H2O
Excretion K+
Excretion Mg2+
Edema
Collagen
deposition
Fibrosis
Arrhythmias
- myocardium
- vessels
ALDOSTERONE INHIBITORS
INDICATIONS
FOR DIURETIC EFFECT
• Pulmonary congestion (dyspnea)
• Systemic congestion (edema)
FOR ELECTROLYTE EFFECTS
• Hypo K+, Hypo Mg+
• Arrhythmias
• Better than K+ supplements
FOR NEUROHORMONAL EFFECTS
• Please see RALES results, N Engl J
Med 1999:341:709-717
ALDOSTERONE INHIBITORS
CONTRAINDICATIONS
• Hyperkalemia
• Severe renal insufficiency
• Metabolic acidosis
Pharmacologic Management
Beta-Blockers
• Cardioprotective effects due to blockade of excessive SNS
stimulation
• In the short-term, beta blocker decreases myocardial
contractility; increase in EF after 1-3 months of use
• Long-term, placebo-controlled trials have shown
symptomatic improvement in patients treated with certain
beta-blockers1
• When combined with conventional HF therapy, betablockers reduce the combined risk of morbidity and
mortality, or disease progression1
1 Hunt, SA, et al ACC/AHA Guidelines for the Evaluation and Management of Chronic
Heart Failure in the Adult, 2001 p. 20.
ß-ADRENERGIC BLOCKERS
POSSIBLE BENEFICIAL EFFECTS
Density of ß1 receptors
Inhibit cardiotoxicity of catecholamines
Neurohormonal activation
HR
Antihypertensive and antianginal
Antiarrhythmic
Antioxidant
Antiproliferative
50
ß BLOCKERS
SURVIVAL
ß Blocker
Placebo
40
30
%
20
10
0
BHAT
JACC 1990;16:1327
< 30%
30-40%
> 40%
LV EJECTION FRACTION
ß-ADRENERGIC BLOCKERS
IDEAL CANDIDATE?
Suspected adrenergic activation
Arrhythmias
Hypertension
Angina
US Carvedilol Study
blockers in
heart failure all-cause mortality
Survival
1.0
Carvedilol
(n=696)
0.9
Placebo
(n=398)
0.8
Risk reduction = 65%
0.7
p<0.001
0.6
0.5
0
50 100 150 200 250 300 350 400
Days
Mortality %
20
Survival
CIBIS-II
1.0
Packer et al (1996)
MERIT-HF
Placebo
Bisoprolol
15
0.8
Metoprolol CR/XL
10
Placebo
Risk reduction = 34%
Risk reduction = 34%
5
0.6
p=0.0062
p<0.0001
0
0
0
200
400
Time after inclusion (days)
600
800
Lancet (1999)
0
3
6
9
12
Months of follow-up
15
18
21
The MERIT-HF Study Group (1999)
Additional benefits of carvedilol in
CHF
• Antioxidant effects
– reduction in myocyte apoptosis
– decreased lipid peroxidation
• Antiproliferative effects
– inhibition of vascular smooth muscle
cell proliferation
• Reduction in circulating endothelin-1
Treatment Approach for the Patient
with Heart Failure
Stage A
Stage B
Stage C
Stage D
At high risk, no
structural disease
Structural heart
disease,
asymptomatic
Structural heart
disease with
prior/current
symptoms of HF
Refractory HF
requiring specialized
interventions
Therapy
Therapy
Therapy
Therapy
• Treat Hypertension
• Treat lipid disorders
• Encourage regular
exercise
• Discourage alcohol
intake
• ACE inhibition
• All measures under
stage A
• All measures under
stage A
• All measures under
stages A,B, and C
• ACE inhibitors in
appropriate patients
Drugs:
• Mechanical assist
devices
• Beta-blockers in
appropriate patients
• Diuretics
• ACE inhibitors
• Beta-blockers
• Digitalis
• Dietary salt restriction
• Heart transplantation
• Continuous (not
intermittent) IV
inotropic infusions for
palliation
• Hospice care
Hunt, SA, et al ACC/AHA Guidelines for the Evaluation and Management of Chronic
Heart Failure in the Adult, 2001
Tratamento da Ins. cardiaca
Inibidores das fosfodiesterases redutores da
produçãode FNT e outras citocinas
Pimobendam, vesnarinona,
pentoxifilina
Ressincronização cardíaca