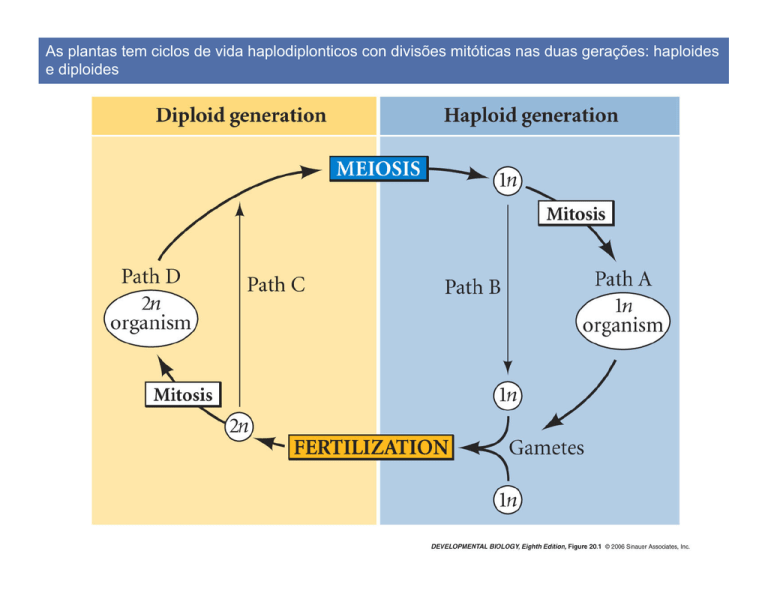
As plantas tem ciclos de vida haplodiplonticos con divisões mitóticas nas duas gerações: haploides
e diploides
Ciclo de vida haplodiplóntico: musgos
Ciclo de vida haplodiplóntico: samambaia
Ciclo de vida de uma angiosperma: planta de ervilhaca (Pisum) parte A
Ciclo de vida de uma angiosperma: planta de ervilhaca (Pisum) (parte B)
(A) Padrões de grãos de pólen (B) Grão de pólen: célula dentro de outra célula
O carpelo consiste do estigma, do estilo, e do ovario com um o varios ovulos
O saco embrionário é o produto de tres divisões mitóticas da megaspore haploide; tem sete
células é oito núcleos haploides
Saco embrionário
Auto-incompatibilidade
Receptor-ligand self-recognition is the key to self-incompatibility in Brassicas
Pollen tube germination
Arabidopsis:
Lírio:
Calcium and pollen tube tip growth
Axis formation in the brown alga Pelvetia compressa
Asymmetrical cell division in brown algae
Angiosperm embryogenesis (parte A) - dicot
Angiosperm embryogenesis (parrte B) - dicot
Radial and axial patterning
An auxin gradient specifies the shoot-root axis (eixo haste-raiz)
The SUS gene (DCL1 allele) suppresses embryonic development in the suspensor
wt
SUS mutant
Dormancy after embryo forms: Viviparous maize mutant
Arabidopsis post germination stages (sporophyte development) (Boyes et al. The Plant Cell, 2001)
(A) STAGE 0.1, IMBIBITION.
(B) STAGE 0.5, RADICLE EMERGENCE.
(C) STAGE 0.7, HYPOCOTYL AND COTYLEDONS
EMERGED FROM SEED COAT.
(D) STAGE 1.0, COTYLEDONS OPENED FULLY.
(E) STAGE 1.02, TWO ROSETTE LEAVES >1 MM IN
LENGTH.
(F) STAGE 1.04, FOUR ROSETTE LEAVES >1 MM IN
LENGTH.
(G) STAGE 1.10, TEN ROSETTE LEAVES >1 MM IN
LENGTH.
(H) STAGE 5.10, FIRST FLOWER BUDS VISIBLE
(INDICATED BY ARROW IN INSET).
(I)
STAGE 6.00, FIRST FLOWER OPEN.
(J) STAGE 6.50, MIDFLOWERING.
(K) STAGE 6.90, FLOWERING COMPLETE.
(L) STAGE 9.70, SENESCENT AND READY FOR SEED
HARVEST.
Both shoots and roots develop from apical meristems, with undifferentiated cells clustered at their
tips
(meristema do caule)
Meristem cells:
Células meristemáticas (Iniciales):
•
•
•
•
Fuente continua de nuevas células.
Isodiametricas
Núcleo Grande
Stem cells
SCR and SHR regulate endodermal differentiation in root radial development (Part 1)
Endoderm
cells
express
SCR
SHR
SCR and SHR regulate endodermal differentiation in root radial development (Part 2)
SCR expression
SCR expression
Organization of the shoot apical meristem
Quimera de tabaco
(L1 y L2 no producen clorofila)
WUS and STM proteins keep meristem cells in an undifferentiated state, while the products of the
CLAVATA genes CLV1, CLV2, and CLV3 limit the number of undifferentiated meristem cells
Polaridad e identidad en la hoja (Husbands et al. 2009 Genes & Development)
Patterning of adaxial and abaxial leaf surfaces of Arabidopsis
Polaridad e identidad en la hoja de Arabidopsis (Husbands et al. 2009 Genes & Development)
Señal de Sussex
derivada del
meristemo induce
lado adaxial en el
primordio de las
hojas (marcación
oscura: PHB,
PHV, REV, etc.)
Polaridad e identidad en la hoja del maiz (Husbands et al. 2009 Genes & Development)
wt
mwp1
lbl1
(miR166)
lbl1
Polaridad e identidad en la hoja (Husbands et al. 2009 Genes & Development)
Leaf morphology mutants in peas
Acacia (tl)
Afilia (af)
af/tl
Simple and compound leaves
KNOX is known to occur in leaf primordia in species making complex leaves (Part 1)
Folha ‘simple’ de Café
KNOX is known to occur in leaf primordia in species making complex leaves
Folha ‘simple’ do arbusto de Amborella
KNOX is known to occur in leaf primordia in species making complex leaves (Parte A)
Folha ‘complexa’ de anis expresa KNOX
Overexpression of Class 1 KNOX genes in tomato
The vegetative-to-reproductive transition (Part 1)
Morphology of a generalized angiosperm sporophyte
20.31 Floral meristem identity mutants
Modelo ABC(D):
https://www.youtube.com/watch?v=Gil3VOQq6k4
Prática – Desenvolvimento em plantas: meristemas
http://vlab.amrita.edu/?sub=3&brch=188&sim=1102&cnt=2
1. Take the onion plant with newly sprouted roots and cut two root tips using scissors and
transfer them into a plastic microfuge tube.
2. Fill 2/3 of the tube with 1N HCl using a dropper.
3. Place the tube in a 60°C water bath and incubate the tube for 12- 15 minutes.
4. Remove the tube from the water bath after the incubation.
5. Discard the HCl from the tube using a Pasture pipette to the running tap water.
6. Add some drops of distilled water into the tube and rinse the root. Then remove the water
from the microfuge tube using the Pasture pipette. (Rinse the roots at least three times).
7. After the washing step add 2-3 drops of Feulgen stain into the tube with root tips and
incubate the roots for 12-15 minutes. (During the incubation, the very tip of the root will
begin to turn red as the DNA stains the numerous small actively dividing cells at the time).
8. After the incubation remove the stain using a Pasture pipette.
9. Again rinse the root tips with distilled water. (Rinse the roots at least three times).
10. Transfer a root from the tube to the centre of the microscopic slide and add a drop of water
over it.
11. Take a razor blade and cut
12. Cover the root tip with a cover slip
13. Observe it under a compound microscope in 10x objective. Scan and narrow down to a
region containing dividing cells and switch to 40x for a better view.
Prática – Desenvolvimento em plantas: meristemas
Prática – Desenvolvimento em plantas








